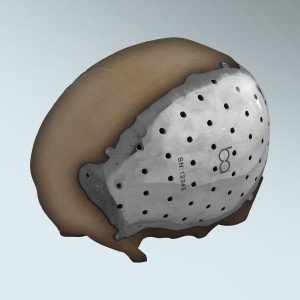
BioArchitects announced today the 510(k) clearance by the U.S. Food and Drug Administration – FDA, for the company’s 3D printed patient specific titanium cranial/craniofacial plate implant. Designed for the repair of defects in the non-loadbearing bones of the head and face, each custom designed plate is permanently attached to the skull and/or face with self-tapping titanium […]

01Feb 2016
16Oct 2015
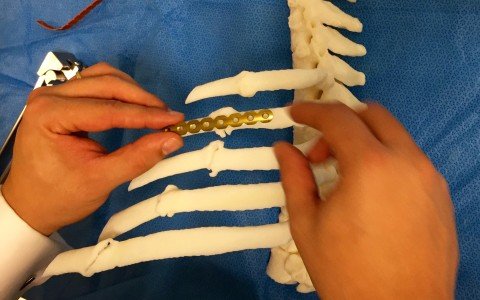
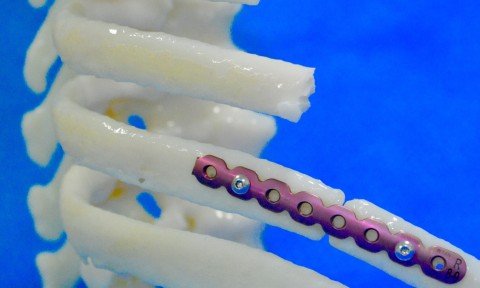
Designed by the Sao Paulo technology company, the anatomical bone model was used for the first time in Brazil in a corrective procedure for multiple fractures of the thorax Accuracy and safety are key factors in medical procedures, and, in recent years, advances in technology have made contributions in these areas. One of the […]
16Oct 2015
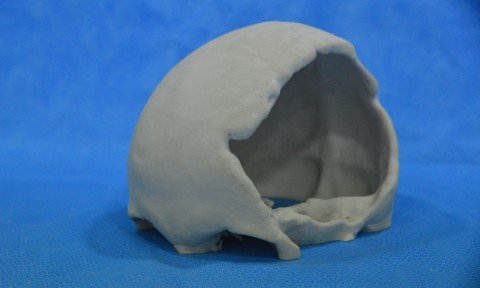
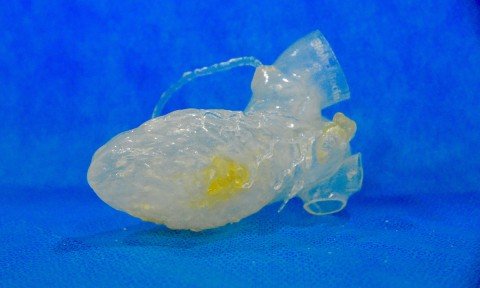
The company, headquartered in Sao Paulo, designs 3D printed human organs in their actual dimensions. Replicas allow doctors to know in advance and in great detail the area of the body being studied and treated, allowing for more precise surgical procedures The use of 3D printing in medicine represents one of the greatest scientific […]